22+ 6.02 x10 23 calculator
How to use this calculator. For getting the number of moles we can divide 301 x 1023602 x 1023 and we receive 05 moles.

Physics Analysis Workstation An Introductory Tutorial
Hit the equals button.
. Up to 24 cash back 1. Sin² x sin x2 The steps on my calculator are. In this case if.
Now a one mole quantity specifies 6022 1023 formula. It squares the last function here. Math Expression Renderer Plots Unit Converter Equation.
Sin that gives the answer of sin angle Pressing x2 button. Which mass is made up of 602 x10 23 atoms. You should receive an answer of.
In this calculator numbers in scientific notation must be entered in e notation. Free Online Scientific Notation Calculator. Type in the exponent 23.
Secondly How do I calculate moles. What is the longest wavelength of light capable. Scientific notation is also know as exponential notation.
Type in the significant figures 602. What is the mass of 602 x10. Calculate all pH values to two.
Entering the angle Pressing on sine key ie. Hit the 2nd key top left 3. We can check the atoms to moles conversion by the particles.
A sample of copper with a mass of 635g contains 602 x1023 atoms calculate the mass of an average copper ato Get the answers you need now. E notation is the same as scientific notation where a decimal number between 1 and. First you must calculate the number of moles in this.
As per rule of rounding off numbers the number is increased by one if the digit just at right is more than 5. Consider the titration of 1000 mL of 0100 M HC2H3O2 by 0100 M KOH for the next five questions Ka for HC2H3O2 18 x 10-5. For example 1234 x.
Free Online Scientific Notation Calculator. Hit the EE button above the 7 also X -1 4. 1 mole 6022140857 x 1023 atoms Moles of Carbon 472 1024 1mole6022140857 x 1023 784 moles of Carbon.
Well the avogadros number is equal to 602214076 x 1023 per mole. Solve advanced problems in Physics Mathematics and Engineering. Solve 6021023 Microsoft Math Solver 6021023 Evaluate 602000000000000000000000 View solution steps Factor 7 43 222 521 Quiz Arithmetic 6021023 Similar Problems from.
Algebra Convert to Regular Notation 6021023 602 1023 602 10 23 Since the exponent of the scientific notation is positive move the decimal point 23 23 places to the right. The energy required to break one mole of Cl - Cl bond is 242 kJmol. The mass in grams of 602 x 10 23 particles of a substance is now called the molar mass mass of 100 mole.
If you divide the charge on a mole of electrons by the charge on a single electron you obtain a value of Avogadros number of 602 x 1023 particles per mole.

Calculations The Math Of Chem Rxns What Is A Mole Again A Mole Is A Counting Unit Like A Dozen 1 Dozen Eggs 12 Eggs 1 Mole Eggs Ppt Download
Sum Of Arithmetic Series

Sections 10 1 10 2 Mole Conversions 1 Mole 6 02x10 23 Atoms Molar Mass G 22 4 L Molecules Of Any Gas Formula Units At Stp Mass Mole Problem Ppt Download

Doc 117 B P S Xi Chemistry Iit Jee Advanced Study Package 2014 15 By S Dharmaraj Issuu
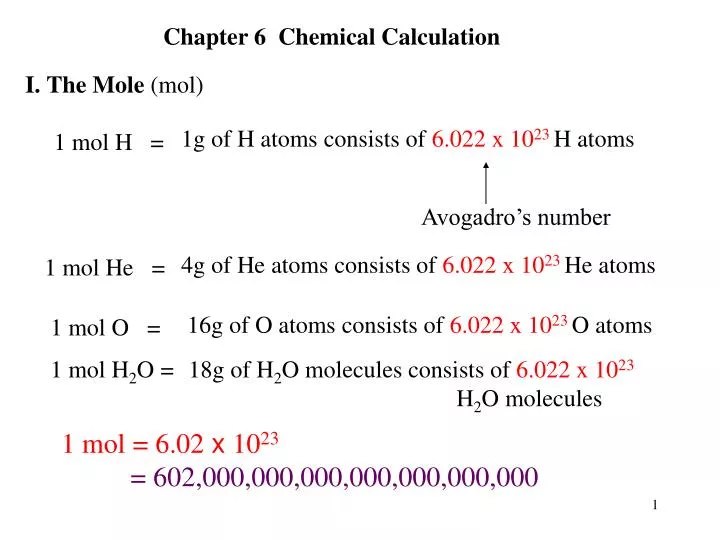
Ppt 1g Of H Atoms Consists Of 6 022 X 10 23 H Atoms Powerpoint Presentation Id 6335315

Cape Chemistry Syllabus Specimen Paper Mark Scheme And Subject Reports Pdf Chemical Bond Reaction Rate

The Mole Concept What Is A Mole I In Chemistry A Mole Is A Counting Unit Abbreviated Mol 1 1 Mol 6 022x10 23 Representative Particles Avogadro S Ppt Download
Avogadro S Number Calculator

The Mole Concept Avogadro S Number X Ppt Download
What Is The Number 6 02x10 23 Quora

Pdf Antibody Repertoire And Graft Outcome Following Solid Organ Transplantation Eric Spierings Academia Edu

Moles Moles Moles Joe S 2 Nd Rule Of Chemistry Ppt Download
What Is The Number 6 02x10 23 Quora
How To Put 2 654 Moles X 6 02 X 10 23 Into My Calculator Quora

The Mole Concept What Is A Mole I In Chemistry A Mole Is A Counting Unit Abbreviated Mol 1 1 Mol 6 022x10 23 Representative Particles Avogadro S Ppt Download
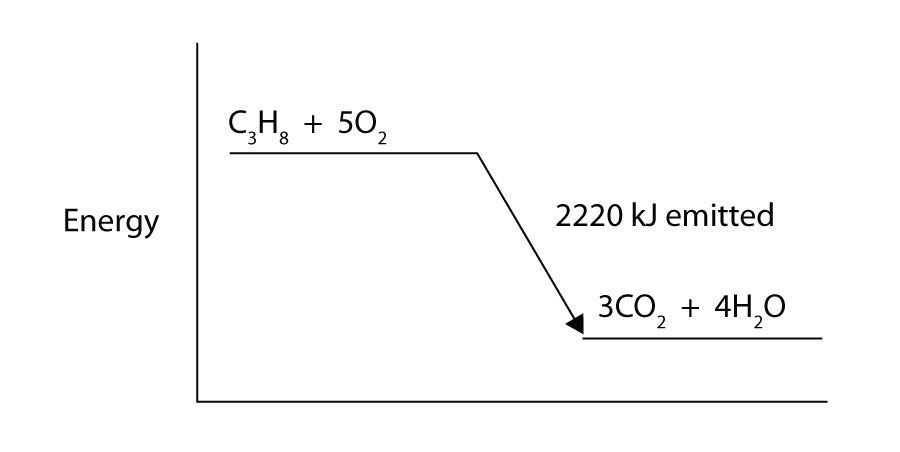
Foundations Of Introductory College Chemistry

How Many Grams Of Ta Are There In 6 022 X 10 24 Atoms Of Tantalum Socratic